In spite of having a cool hat (and a great tutu)
this guy never did figure out how to turn lead
into gold. He should have asked a physicist.
As we look out into the Universe and identify the many accidents of physics and
astronomy that have worked together to our benefit, it almost seems as if the
Universe must in some sense have known that we were coming. — Freeman Dyson
Fusion (For Laycreatures)
To make sense of the rest of this, I’ll briefly explain how nuclear fusion works. The simplest explanation is that you’re “squeezing” smaller atoms together to make a different, bigger atom.
But the heart of a star is so hot that the electrons are stripped off. The actual fusion takes place between the nuclei — the remaining clumps of protons and neutrons.
The heart of a star is kind of like a mosh pit. If the temperature is high enough, these nuclei become so energetic, they start dancing and coming very close to one another. Since like charges repel, the protons want to keep them apart.
But enter the Strong Nuclear Force (which is mediated by gluons). If two nuclei come very close together, very quickly, the strong force will overcome the repulsion and the nuclei will “tunnel” together. (Kind of like a shotgun wedding, but without the preacher and firearms.) Y’gets a new element.
 Like this, but a lot smaller.
Like this, but a lot smaller.
(And with more intelligence.)
Fast-Forward …
OK, let’s pick up where we left off last time. We’ll fast forward to about 380,000 years after the Big Bang. By this time, the universe has cooled enough for electrons to marry up with the nuclei created in the first minute, creating h’actual h’atoms of hydrogen (and some helium, with a smidge of lithium just to keep everyone happy).
This was the original Interstellar Medium (or “ISM,” if you’re an Astro-Person): essentially just a slowly expanding cloud of hydrogen. Over millions and billions of years, gravity formed clumps that drifted together, then flattened out into gigantic, slowly spinning disks called galaxies. Local concentrations of hydrogen clumped to form stars.
The universe was a lot different back then. When the first radio telescopes were built, astronomers were freaked out by some extremely distant and unbelievably powerful signals. The Astro folks called them Quasi-Stellar Radio Sources: quasars (see below). They were eventually able to eyeball one of these and realized that it was just a huge, ancient galaxy with a monstrous black hole in the middle.
Here’s a cool picture called Einstein’s Cross. The cloud in the center is a galaxy called “Huchra’s Lens,” about 400 million light years from Earth. The four bright dots are a quasar directly behind it, 8 billion light years away. Huchra’s gravity bends the light from the quasar into 4 distinct images.
Or maybe it’s a UFO landing on your head.
“Elements, My Dear Watson!”
Now let’s take a look at the elements that you had to memorize when you learned the Periodic Table (I’ve also posted an image below) in science class.
The number of protons is what determines an element’s identity. For example, hydrogen has a single proton; it’s a flammable gas. If you add a second proton, you get helium, which is a gas, but is not flammable. Add a third proton and you get lithium, which is a metal. And so on.
(I don’t need to bring neutrons in at this point. Maybe later. Seriously, the number of protons is what “defines” the element.)
By the late 1950’s, physicists had a good idea of how these elements were created through nuclear fusion. Fred Hoyle, working with Willy Fowler and the Burbidges, Geoffrey and Margaret , developed the theory of stellar nucleosynthesis: “heavier” elements were created in stars.
But there is a huge difference in the abundances of each element. It’s not hard to guess why there’d be more iron (“Fe” in the illustration at that link) than gold (“Au”); the precious metal is a larger and “heavier” atom. But why is there so much more sulfur (“S”) than fluorine (“F”)? The stinky yellow stuff actually has a “heavier” atom than the stinky gas!
It turns out that this is written into the laws of nature, which I’ll cover next. Yes, it’s another fine-tuning thingie — another “cosmic coincidence.”
 When a star is born, the radiation pressure
When a star is born, the radiation pressure
blows away the dust so that you can see it.
(Strike up, “You’re So Vain…”)
“I Will Love Him And Hug Him And Call Him George!”
As usual, I’m providing a brief, not-strictly-accurate overview. My examples will be limited to two stars: our Sun and a typical red supergiant. There are zillions of details and other cases that I must leave out here. For example, if you look at that periodic table below, you’ll see that elements can be created in different ways; I’m oversimplifying. (You can thank me later.)
But I’ve already alluded to this: high pressure means a high temperature. When you see a shooting star, that meteor burns up because it compresses the atmosphere in front of it. (Note to my friends in the media: it’s not “air friction!”)
Out in space, some of the ISM is squeezed into a ball by gravity. If the temperature in the center — the “core” — goes high enough, you get fusion: hydrogen is “burned” into helium. When we do this on the small scale here on Earth, we call it a “hydrogen bomb.” On the large scale, out in the Cosmos, we say, “a star is born.”
Gravity wants to keep hugging and squeezing and calling the core “George,” but the fusion of lighter elements releases a lot of energy. If George is squeezed too hard, more fusion occurs, which pushes back against gravity. If the fusion drops off, gravity squeezes harder, so the fusion ramps back up. A nice, self-regulating balance is reached.
(The geeks call this “hydrostatic equilibrium,” for those who like fancy terms.)
Sirius A and B. Just like your brother eats all the chips,
Sirius A is a pig who gobbled up most of the hydrogen.
Resonances
The temperature determines which elements will be fused. Heavier elements require a hotter core, but release less energy when they fuse. (Hold that thought; it’s important.)
Once a star’s core burns all of a given element, the fusion tapers off. Gravity can say, “I love you, George!” and squeeze harder, ratcheting up the temperature. The star finds a new (and denser) equilibrium, “burning” a heavier element.
OK, so we start by fusing hydrogen into helium. Now what? The next element in the periodic table is lithium, but it’s rare in nature. Maybe beryllium? Nah, that’s uncommon as well.
This was actually one of the first “fine tuning” thingies to be discovered. Fred Hoyle once famously joked that his atheism had been “badly shaken” by what he’d learned. This particular resonance is called the “Hoyle resonance” in his honor.
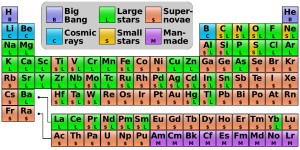 Where every element comes from.
Where every element comes from.
Not shown: the origin of the stuff
that Aunt Petunia uses on her hair.
![]() Back to the “mosh pit” at the center of that star. Just as a crowd goes wild when some Slayer or Cannibal Corpse* starts blasting, at certain temperatures, some atoms (well, nuclei) become insanely excited. They’re far more likely to fuse at certain specific temperatures.
Back to the “mosh pit” at the center of that star. Just as a crowd goes wild when some Slayer or Cannibal Corpse* starts blasting, at certain temperatures, some atoms (well, nuclei) become insanely excited. They’re far more likely to fuse at certain specific temperatures.
By a happy “coincidence,” as stars age and the core becomes hotter, step by step, they precisely hit or miss these “resonant” temperatures as needed for life chemistry.
For example, gobs of carbon will be made because of a specific resonance (see the Triple Alpha Process illustration below). But later on, the star’s temperature is just far enough off of resonance to make plenty of oxygen — without destroying all of the carbon that was created previously. The bottom line is that we end up with plenty of CHON — Carbon, Hydrogen, Oxygen and Nitrogen — the key elements for life as we know it.
Why, it’s almost as if the universe knew we were coming! (Freeman Dyson said so.)
The Triple-Alpha Process: Beryllium (8Be in the image)
is created, but a resonance marries it to another 4He
(helium), creating a nucleus of carbon (12C) instead.
They live happily ever after and we get lots of carbon.
Objections (And Answers)
We met Victor Stenger in the previous section. He argues, borrowing from Steven Weinberg, that this particular resonance isn’t that big of a deal.
But remember what I said about keeping up with this stuff? You can’t just read a dismissal of one of these “fine-tuning” thingies and consider it settled. The clear trend is toward more evidence of fine-tuning as new discoveries are made, not less.
I mentioned Luke Barne’s critic of the Fallacy of Fine Tuning above. It’s a PDF, but you may click that link, open the PDF, then search for “Resonance” in that paper. Basically, translating what Barnes says into laycreature-language, for life as we know it in our Cosmos and based on what we know now, this resonance must be what it is to within .4%. (That’s point 4 percent.)
Let’s revisit another objection. Some skeptics (including Stenger) complain that you can’t just “adjust one force” and then be amazed that it makes life less likely. As proof of what I said — that this not only doesn’t help them, it actually makes it worse — Barnes points out that when the fine structure constant (which I’m not about to try to explain here; click the link for more info) is added to the mix, this becomes even more critical.
The fine structure constant’s value must be within .001% of what we observe, or the “Hoyle resonance” wouldn’t help us at all. Stars would either make gobs of carbon or gobs of oxygen — not both. We wouldn’t be here.
Jaleel “Jim” Al-Khalili, OBE, BSc, PhD. Theoretical
Physicist. Atheist, President of the British Humanist
Association. Believes our Cosmos is fine-tuned for life.
Small Star: Him Get Old, Shed Weight
All stars consume themselves as they age, fusing lighter into heavier elements. The larger the star, the more quickly it burns itself up. Our Sun is small enough that it will continue burning hydrogen to helium for billions of years. Betelgeuse in the constellation Orion, on the other hand, is a red supergiant. It has at least 8 times the mass of our own Sun and won’t live beyond 10-15 million years.
In fact, it may be nearing the end of its life. The ancient Chinese recorded that Betelgeuse was yellow, but now it’s red. Its core has long since stopped burning hydrogen to helium and is working on heavier elements. If those old records are right, Betelgeuse has changed visibly in just a few thousand years(!).
Back to our Sun. Billions of years from now, it will swell and cool. Earth will be swallowed and eaten, joining George down in the center. Eventually, the core will finally run out of fuel for fusion. The Sun just isn’t large enough for gravity to squeeze any harder, so it dies with a sigh: it will shed its outer layers and become a white dwarf star.
The outer layers drift away, adding a bit of carbon and oxygen to the ISM … and we get a beautiful Planetary Nebula (a misleading name that was given to them back in the late 1700’s, but we’re stuck with it now).
The Necklace Nebula happened when one star swelled
up and ate another star. The white dot in the middle
is two remnant white dwarves orbiting one another.
Beetlejuice … erm, Betelgeuse. He’s not dead (yet),
but he is a big fellow. If our Sun was edited into that
image to scale, it would be smaller than a single pixel.
Big Star: Him Get Old, Go BOOM
To get heavier elements, you need a heavy star like Betelgeuse. He might blow off an outer layer from time to time, but the main event will come at the end of his life. He’s expected to explode violently as a supernova.
OK: as the star ages, the core becomes choked with a given element. Fusion tapers off, gravity yells, “George!” and hugs and squeezes harder, raising the temperature. Another, heavier element is fused. The lighter elements drift up, away from the core. In time, the star becomes layered like an onion with different elements. See the left image below (click for a larger picture).

Left: what the inside of Betelgeuse might look like just before
going “boom.” Right: a Chandra X-ray image of a supernova.
The color-coding is added to show that it blew itself inside out.
Sometime in the future: Betelgeuse eventually reaches the point where the core is choked with iron. It takes more energy to fuse iron than you can get from the reaction, so gravity pulls more material into the center. The star is now dying. Once the core reaches about 1.44 times the mass of our Sun, that’s the straw that breaks the back: Betelgeuse kills itself. The core collapses into a ball of neutrons. Zillions and zillions and zillions of neutrinos are released.
In the meantime, with nothing to hold up the outer layers, gravity happily screams, “GEORGE!” and reaches out in a final loving hug and squeeze. He yanks the outer layers toward the core so hard, they’re traveling as fast as 23% of the speed of light(!!) when they get there … and meet that outward-bound “wall cloud” of neutrinos. All of the heavy elements through Uranium are created in one final cataclysm.
The same Weak Force that worked in the Big Bang to give us the right mix of protons and neutrons also governs those neutrinos. It is “fine tuned” to create and then push these fresh-baked elements into the Cosmos. All that’s left behind is a spinning remnant neutron core (a pulsar) and a faint, distraught voice: “George …”
Left: The Crab Nebula in visible light. Right: a Chandra
X-ray image of the spinning remnant pulsar in the center.
Setting The Stage
By 9-10 billion years after the Big Bang, the Interstellar Medium has been enriched with heavier elements. We have iron, silicon, oxygen, carbon and stinky sulfur, ready to form planets and people who can argue that it all Just Happened(tm) and No Big Deal(tm).
From time to time, a spinning cloud of this rich dust will form a protoplanetary disk. One or more stars will be born in the center, while the circling cloud of dust “clumps” into planets.
Better telescopes have allowed us to directly eyeball how planets might form. Have a look at the images below. The one on the left is from the Orion Nebula, 1000-1300 light years away. The other is around the star designated HD 107146, 88 light years from Earth.
This is another case of, “we know more now …” and we’re going to cover that next.
Note the little baby star glowing red in the
disk to the left. On the right, the star has
been blacked out to reveal the disk.
Use the menu on the left, or proceed to the next section, “A Home For Life.”
*A warning for tender hearts and sensitive ears: don’t go searching for “Cannibal Corpse” on YouTube. Your eyes will start smoking and your hair will fall out.